Haloarenes and Method of preparation of Haloarenes
Haloarenes are the halogen derivatives of aromatic hydrocarbons in which halogen is directly attached to the ring. these are obtained by replacing hydrogen by alpha-halogen atom.$$Ar-H\xrightarrow{-H\,/+X}Ar-X$$ $$Where\,Ar-H=Aromatic\,substution$$
Summary
Haloarenes are the halogen derivatives of aromatic hydrocarbons in which halogen is directly attached to the ring. these are obtained by replacing hydrogen by alpha-halogen atom.$$Ar-H\xrightarrow{-H\,/+X}Ar-X$$ $$Where\,Ar-H=Aromatic\,substution$$
Things to Remember
- Haloarenes are the halogen derivatives of aromatic hydrocarbons in which halogen is directly attached to the ring. these are obtained by replacing hydrogen by alpha-halogen atom.
-
Aromatic hydrocarbon is hydrogenated directly at dark in presence of Lewis acid such as AlCl3, FeCl3, FeBr3 to produce corresponding haloalkanes.
- To get chlorobenzene from aniline,aniline is first converted into benzene diazonium chloride.
MCQs
No MCQs found.
Subjective Questions
No subjective questions found.
Videos
No videos found.

Haloarenes and Method of preparation of Haloarenes
Haloarenes
Haloarenes are the halogen derivatives of aromatic hydrocarbons in which halogen is directly attached to the ring. these are obtained by replacing hydrogen by alpha-halogen atom.
$$Ar-H\xrightarrow{-H\,/+X}Ar-X$$
$$Where\,Ar-H=Aromatic\,substution$$
$$Ar-X=Haloarene$$
Nomenclature.
Monosubstituted haloarenes.
.png)
1.png)
A general method for preparation of Haloarenes.
1. By direct halogenation of aromatic hydrocarbon.
$$Ar-H+X(Cl\,orBr)\xrightarrow{lewis\,acid\,dark}Ar-X+HX$$
$$Ar-H=Aromatic\,hydrocarbon$$
$$Ar-X=Haloarenes$$
Aromatic hydrocarbon is hydrogenated directly at dark in presence of Lewis acid such as AlCl3, FeCl3, FeBr3 to produce corresponding haloalkanes.

The reaction of an aromatic hydrocarbon with iodine is reversible. Therefore, iodination is carried out in presence of oxidation agent like HNO3, HIO3 etc. with accelerate the rate of forward reaction.

$$HIO_3+5HI→3H_2O+3I_2$$
2. From diazonium salts.
Diazonium salts. is obtained by adding the ice-cold solution of NaNO2 slowly on aniline in HCl. This reaction is Known as diazotization.
$$NaNO_2+HCl\xrightarrow{<5^0C}HNO_2+NaCl$$
$$Where\,HNO_2=Nitrous\,acid$$
Preparation of Chlorobenzene
1. From benzene.
When benzene is chlorinated directly with Cl2 in presence of FeCl3 at room temperature chlorobenzene is obtained.
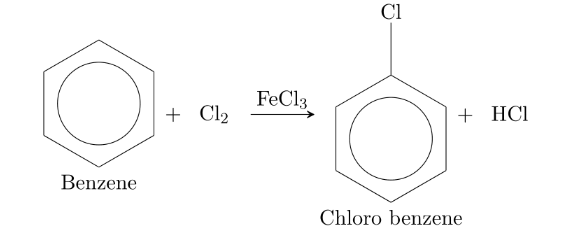
2. From Aniline.
To get chlorobenzene from aniline,aniline is first converted into benzene diazonium chloride.
The product thus obtained is then reacted with Cu2Cl2 in presence of HCl to get chlorobenzene.

3. From phenol.
For that, phenol is first refluxed with Zn-dust to get benzene.

Then chlorination of benzene is carried out as to get chlorobenzene.

Preparation of chloroand bromobenzene (Sandmeyers reaction).
When benzene diazonium chloride is treated with (Cu2Cl2+HCl) and (Cu2Br2+HBr)separately. corresponding chlorobenzene and bromobenzene are formed. This reaction is called Sandmeyers reaction.

Preparation of Iodobenzene.
Benzene in presence of I2 and HNO3 give IOdobenzene
.png)
Iodobenzene is obtained by adding aueousKI solution to benzene diazonium chloride.

Preperation of fluoro benzene (Balz-schiemann reaction)


Reference.
Bahl, B S, Bahl, and Arun. Advanced Organic chemistry. S. Chand and company Ltd., n.d.
Sthapit, M K, R R Pradhananga, and K B Bajracharya. Foundations of chemistry. Taleju Prakashan, n.d.
Tewari, K S, S N Mehrotra, and N K Vishnoi. A textbook of organic chemistry. Vikash publishing House Pvt. ltd., n.d.
Verma, N K and S K Khanna. Compressive chemistry. 8th edition. Laxmi publications P. Ltd., 1999.
Lesson
Haloalkanes and Haloarenes
Subject
Chemistry
Grade
Grade 12
Recent Notes
No recent notes.
Related Notes
No related notes.