Victor Meyers method of distinguish Primary,Secondary and Tertiary alcohol
Victor Mayer method is one of the most important methods of identification of alcohols. In this methods primary,secondary and tertiary alcohols are subjected to a series of chemical analysis and the colour of resulting solution is observed. A primary alcohol gives blood red colour. secondary alcohol gives the blue colour. A tertiary alcohol does not produce any colour.
Summary
Victor Mayer method is one of the most important methods of identification of alcohols. In this methods primary,secondary and tertiary alcohols are subjected to a series of chemical analysis and the colour of resulting solution is observed. A primary alcohol gives blood red colour. secondary alcohol gives the blue colour. A tertiary alcohol does not produce any colour.
Things to Remember
-
A primary alcohol gives blood red colour.
b. A secondary alcohol gives the blue colour.
c. A tertiary alcohol does not produce any colour.
MCQs
No MCQs found.
Subjective Questions
No subjective questions found.
Videos
No videos found.

Victor Meyers method of distinguish Primary,Secondary and Tertiary alcohol
Victor Meyers method of distinguishing Primary,Secondary and Tertiary alcohol
Victor Mayer method is one of the most important methods of identification of alcohols. In this methods primary,secondary and tertiary alcohols are subjected to a series of chemical analysis and the colour of resulting solution is observed.
The different steps involved in Victor Meyer methods are as below
- The alcohol is treated with iodine in presence of red phosphorous to obtain iodol alkane.
- Iodoalkane so formed is allow to react with alcoholic silver nitrate in order to obtained nitroalkane.
- The nitroalkane is treated with nitrous acid ( the mixture of Nano2 +HCl) and the resulting solution is made alkaline.
- the colour of resulting solution is observed in which following observations are observed.
a. A primary alcohol gives blood red colour.
b. A secondary alcohol gives the blue colour.
c. A tertiary alcohol does not produce any colour.
Following reactions are involved in the victor may methods.
primary methods.
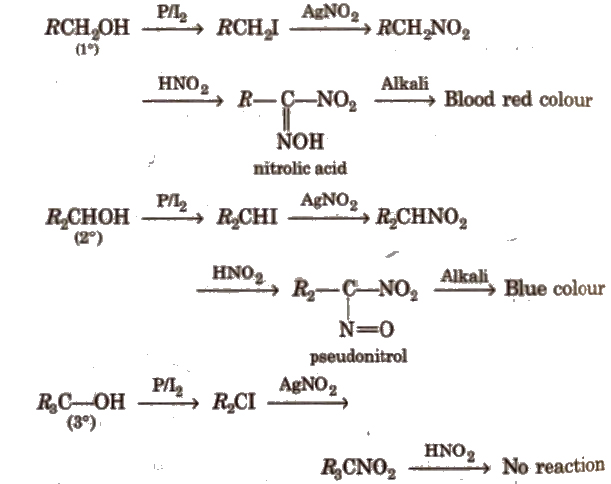
In this way, Primary,Secondary, and Tertiary alcohols can be identified by Victor Meyer method.
An organic compound 'A; hives "B" on photochlorination. Compound "B"reacts with sodium cyanide to produce "C" which on treatment with LIAH forms "D" when compound D is treated ith nitrous acid, compound E is obtained . A can also be obtained by treating chloromethane with sodium in presence of dry ether. Identify A, B, C , D, E . Given sequence of reaction can be represented as;
Given sequence of reaction can be represented as.
$$A\xrightarrow{Cl_2\,hv}B\xrightarrow{NaCN}C\xrightarrow{LiAlH_4}D\xrightarrow{HNO_2}E$$
Chloromethane reacts with sodium in dry ether to produce ethane which is compound A.
$$\underbrace{CH_3Cl}_{Chloro\,methane}+Na+Cl-CH_3\xrightarrow{dry\,ether}\underbrace{CH_3-CH_3}_{Ethane\,(A)}+NaCl$$
Ethane further undergoes photochlorination to give chloroethane which is compound B.
$$\underbrace{CH_3-CH_3}_{Ethane}+Cl_2\xrightarrow{Cl_2\,hv}\underbrace{CH_3-CH_2-Cl}_{Chloro\,ethane\,(B)}+HCl$$
Since chloroethane reacts ith sodium cyanide to form propane nitrile, which is compound C.
$$\underbrace{CH_3-CH_2-Cl}_{Chloro\,ethane\,}+NaCN\xrightarrow{alcohol}\underbrace{CH_3-CH_2-CN}_{Propanenitrile\,(C)}+NaCl$$
propane nitrile is reduced by LiALH to form 1-propanamine which is compound D.
$$\underbrace{CH_3-CH_2-CN}_{Propanenitrile}\xrightarrow{LiAlH_4}\underbrace{CH_3-CH_2-CH_2-NH_2}_{1-Propanamine\,(D)}$$
1-propanamine reacts with nitrous acid to produce 1-propanol which is compound E.
$$ \underbrace{CH_3-CH_2-CH_2-NH_2}_{1-Propanamine}+HNO_2\xrightarrow{NaNO_2+HCl\,<5^0C}\underbrace{CH_3-CH_2-CH_2-OH}_{1-Propanol}+N_2+H_2O$$
Now the overall reaction can be written as;
$$\underbrace{CH_3-Cl}_{Chloro\,methsane}\xrightarrow{dry\,ether}\underbrace{CH_3-CH_3}_{A}\xrightarrow{Cl_2\,hv}\underbrace{CH_3-CH_2-Cl}_{B}\xrightarrow{NaCN}\underbrace{CH_3CH_2-CN}_{C}\xrightarrow{LiAlH_4}\underbrace{CH_3-CH_2-CH_2-NH_2}_{D}\xrightarrow{HNO_2}\underbrace{CH_3-CH_2-CH_2-OH}_{E}$$
Hence A,B,C,D and E are
$$A:\underbrace{CH_3-CH_3}_{Ethane}$$
$$B:\underbrace{CH_3-CH_2-Cl}_{Chloro\,ethane}$$
$$C:\underbrace{CH_3CH_2-CN}_{Propanenitrile}$$
$$D:\underbrace{CH_3-CH_2-CH_2-NH_2}_{1-Propanamine}$$
$$E=\underbrace{CH_3-CH_2-CH_2-OH}_{1-Propanol}$$
Reference.
Bahl, B S, Bahl, and Arun. Advanced Organic chemistry. S. Chand and company Ltd., n.d.
Sthapit, M K, R R Pradhananga, and K B Bajracharya. Foundations of chemistry. Taleju Prakashan, n.d.
Tewari, K S, S N Mehrotra, and N K Vishnoi. A textbook of organic chemistry. Vikash publishing House Pvt. ltd., n.d.
Verma, N K and S K Khanna. Compressive chemistry. 8th edition. Laxmi publications P. Ltd., 1999.
Lesson
Alcohols and Phenols
Subject
Chemistry
Grade
Grade 12
Recent Notes
No recent notes.
Related Notes
No related notes.