Physical property of alcohol
Alcohol can form the hydrogen bond with the water molecule. Therefore, they are soluble in polar solvents like water. Their solubility , however, decreases with increase in a size of an alkyl group.Ethene is oxidised in the air in presence of catalyst and temperature to produce epoxy ethane which on acid hydrolysis gives ethylene glycol.Fats and oils are the esters of long chain fatty acids known as triglycerides. These esters can be hydrolyzed both in acidic and basic medium to manufacture glycerol. Alkali hydrolysis of these esters produces glycerol and soap. This process is known as saponification.
Summary
Alcohol can form the hydrogen bond with the water molecule. Therefore, they are soluble in polar solvents like water. Their solubility , however, decreases with increase in a size of an alkyl group.Ethene is oxidised in the air in presence of catalyst and temperature to produce epoxy ethane which on acid hydrolysis gives ethylene glycol.Fats and oils are the esters of long chain fatty acids known as triglycerides. These esters can be hydrolyzed both in acidic and basic medium to manufacture glycerol. Alkali hydrolysis of these esters produces glycerol and soap. This process is known as saponification.
Things to Remember
- Alcohol can form the hydrogen bond with the water molecule. Therefore, they are soluble in polar solvents like water. Their solubility , however, decreases with increase in a size of an alkyl group.
-
when ethene is oxidised by Bayer reagent , Ethylene glycol is formed .
-
Glycerol in presence of PCl5 form 1,2,3 -trichloro propane
MCQs
No MCQs found.
Subjective Questions
No subjective questions found.
Videos
No videos found.

Physical property of alcohol
The physical property of alcohol.
Some of the physical property of alcohol are as follows.
- Solubility.
- Boiling point.
Solubility.
Alcohol can form the hydrogen bond with the water molecule. Therefore, they are soluble in polar solvents like water. Their solubility , however, decreases with increase in a size of an alkyl group.
The solubility of alcohols is higher than these of isomeric haloalkanes ,ether etc.
Boiling point.
The presence of polar -OH groups in alcohols permits the formation of intermolecular H-bond among alcohols molecules .
As a result, alcohols boil at the higher temperature than corresponding haloalkanes ,ethers etc.
Dihydric alcohol: Ethylene glycol.
Structure.
*Preparation:
a) from ethene,
when ethene is oxidised by Bayer reagent , Ethylene glycol is formed .

b) From epoxy compound:
Ethene is oxidised in the air in presence of catalyst and temperature to produce epoxy ethane which on acid hydrolysis gives ethylene glycol.
Chemical reactions.
Uses
1) As antifreeze in automobile radiators and aeroplane motors.
2) A solvent for storing ink.
3) Nitroglycol prepared from it can be used as an explosive.
4) In the manufacture of polymer, Bacron.
Trihydric alcohol : :Glyceral
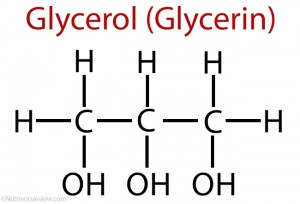
Preparation:
From oils and fats:

Fats and oils are the esters of long chain fatty acids known as triglycerides. These esters can be hydrolyzed both in acidic and basic medium to manufacture glycerol. Alkali hydrolysis of these esters produces glycerol and soap. This process is known as saponification.

Reactions:
1. Reaction with PCl5.
Glycerol in presence of PCl5 form 1,2,3 -trichloro propane
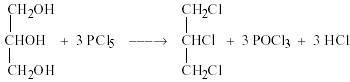
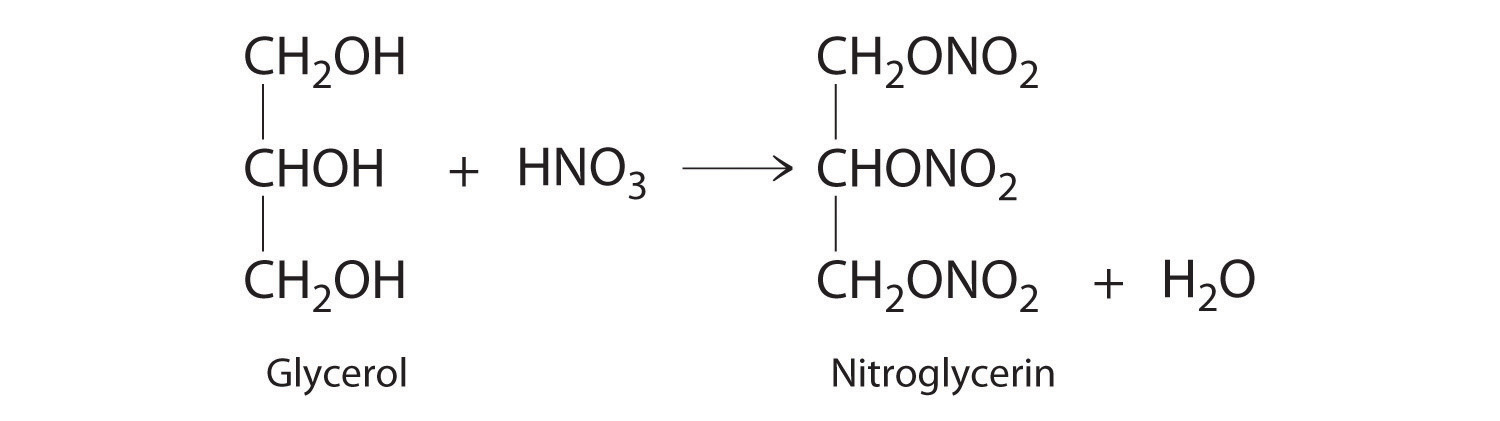
2. Reaction in presence of Conc HNO3. form Nitroglycerine
3. Reaction in presence of HI
Glycerol in presence of HI forms 1,2,3 Tri-iodopropane

Uses;
a) Manufacturer of nitroglycerine an important explosive.
b) Used as a sweetening agent in beverages.
c) As an antifreeze in automobile radiations.
d) As a lubricant for watches and clock.
e) In the manufacture of cosmetics and high quality soaps.
Reference.
Bahl, B S, Bahl, and Arun.Advanced Organic chemistry.S. Chand and company Ltd., n.d.
Sthapit, M K, R R Pradhananga, and K B Bajracharya.Foundations of chemistry.Taleju Prakashan, n.d.
Tewari, K S, S N Mehrotra, and N K Vishnoi.A textbook of organic chemistry.Vikash publishing House Pvt. ltd., n.d.
Verma, N K and S K Khanna.Compressive chemistry.8th edition. Laxmi publications P. Ltd., 1999.
Lesson
Alcohols and Phenols
Subject
Chemistry
Grade
Grade 12
Recent Notes
No recent notes.
Related Notes
No related notes.